Express your interest in the trial by completing the online reply slip
This information sheet is intended to provide summary information regarding the ECAL trial. Please refer to the Main Information Sheet for a more detailed description of the trial intervention arms, procedures, risks, and information on how your data will be stored and used. The Main Information Sheet can be found at: Main Participant Information Sheet (711KB)
This information sheet is provided in English. Birmingham Clinical Trials Unit (BCTU) can help with language translation if needed. If you would like access to a translation service, please contact ECAL@trials.bham.ac.uk
You are being invited to consider whether you would like to take part in a research trial. Before you decide, it is important for you to understand why the research is being done and what it will involve. Please take time to read the following information carefully and discuss it with others or a member of the ECAL trial team.
What is the purpose of the trial?
About a third of people diagnosed with chronic obstructive pulmonary disease (COPD) smoke cigarettes. It is important that people with COPD who smoke are supported to stop as continued smoking speeds up the progression of the disease and importantly stopping smoking slows it down. Our goal with this research is to find out how effective electronic cigarettes (EC) are compared with combination nicotine replacement therapy (NRT) in helping people with COPD to quit smoking cigarettes, and which product is more cost-effective for the National Health Service (NHS). We are also investigating how switching to an EC affects lung health and wellbeing.
Who are we?
This trial is run by the University of Birmingham and Queen Mary University of London, and it is funded by the National Institute for Health Research.
Is this trial for me?
We are looking for people aged 35 years and older who have been diagnosed with COPD who are current smokers and who want to try to quit smoking within 2 weeks of starting the trial. If you are not a current smoker, please ignore this invitation.
If you think you might be interested in taking part, you can express an interest by returning a reply slip (as outlined on your invite) or contacting the ECAL trial team. Expressing an interest is not a commitment to take part in this trial. We would also still need to check your eligibility to see if you can take part.
Trial products
In this trial, participants will either be given an electronic cigarettes (ECs) starter kit with an initial supply of e-liquid or up to 12 weeks supply of combination nicotine replacement therapy (e.g., nicotine patch + nicotine gum) to help them quit smoking. The research team will describe how to use these products, any potential side effects and explain any additional procedures required. These will be explained in person and are also detailed in the main ECAL Participant Information Sheet provided.
What happens in the trial?
If you express an interest to take part, a member of the ECAL trial team will phone you to go through pre-baseline questions to check you are suitable for a baseline visit. If you are, we will invite you to the nearest participating research site for a face-to-face visit. During this visit we will ask you to sign a consent form, to confirm information about your medical history and your current health, and you will be asked to complete a questionnaire. You will also be asked to give a blood sample, undergo spirometry and a breath test to measure carbon monoxide levels. All participants enrolled in the trial will be given either an EC or combination NRT and will receive weekly calls over a period of approx. 6-8 weeks from a trained stop smoking advisor. All participants will be followed up at 4 weeks and 6 months by questionnaire, and again at 12 months in a face-to-face visit at your nearest participating research site. At the 12-month visit, you will be asked again to provide a blood sample, to undergo spirometry, to undergo the carbon monoxide breath test, to answer questions about your health and to fill out a questionnaire.
Below is a summary of what will happen as part of the trial:
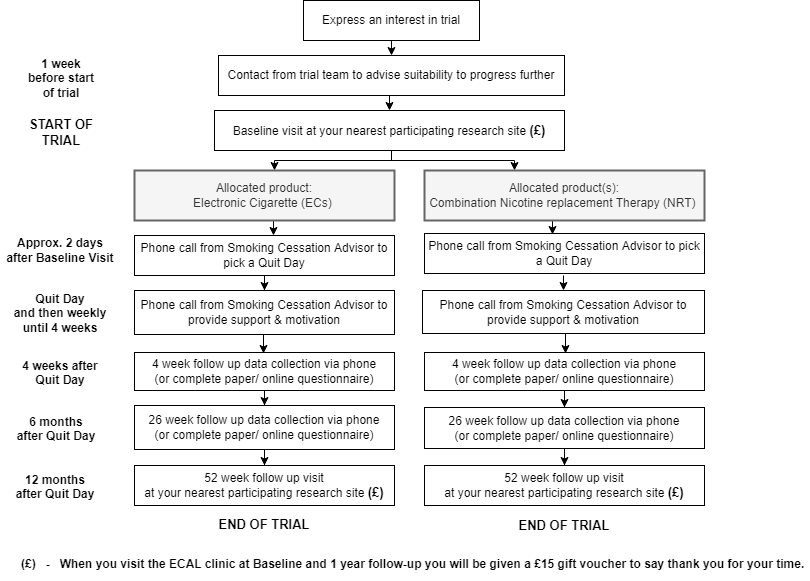
How will it be decided who gets which products?
If you decide to take part in the trial, and you meet the eligibility requirements, the research team will randomly allocate you (by a computer) to one of the trial products (either EC or Combination NRT). The trial products will be given in addition to any usual care you normally receive for your COPD.
Do I have to take part?
No, this is entirely up to you. Taking part is voluntary. You do not have to take part if you do not want to. It will not affect your standard care in any way. There is a £15 gift voucher for your time after the first visit and a further £15 voucher at the final 12-month visit.
Disadvantages
The trial products are already in common use in the UK and are considered safer than cigarette smoking. They may, however, cause some side effects. These are mostly mild and detailed in the main information sheet. Other disadvantages may include having to provide blood samples. Blood sample taking will be carried out by experienced doctors and nurses.
All samples taken and data will be kept confidential.
You can withdraw from the trial at any point and do not have to provide a reason.
Who can I contact for further information?
If you would like to know more about this trial, or have any questions, please contact the ECAL trial team at Birmingham Clinical Trials Unit by email at ECAL@trials.bham.ac.uk
Express your interest in the trial by completing the online reply slip



IRAS ID: 1006828 ISRCTN: 82413824 ECAL Trial _PIS Summary Sheet_v2.0_18_Jul_2023