Research in the Molecular Synthesis Section focusses on answering fundamental questions relating to molecular structure, chemical reactivity and physical properties enabled by the development of cutting edge synthetic methodology. These enabling methods allow us to design bespoke functional molecules as novel chemical entities with the potential to tackle major societal challenges, spanning sustainability (energy, chemical feedstock, agriculture) and next generation therapeutics and diagnostics, or indeed any area where new molecules can answer scientific problems. To deliver these impacts, we collaborate widely both with the University of Birmingham and (inter)nationally. Our research is supported by the Birmingham Centre for Chemical and Materials Analysis, which offers high quality spectroscopic analysis, including NMR, Mass Spectrometry, X-ray Diffraction and HPLC.
Areas of expertise:
Synthetic coordination, main group, organic, organometallic and supramolecular chemistry for functional molecules; catalytic methodology design and discovery; mechanical bonding, molecular diversity and scaffold design; physical organic chemistry and reaction mechanisms; stereoselective synthesis; sustainability; cages; carbohydrates; catenanes; foldamers; lipids; natural products; peptides; rotaxanes; synthetic DNA.
Synthesis Section Lead
Areas of interest: Rotaxanes and Catenanes – Stereochemistry – Prodrugs – Coordination Chemistry – Catalysis
Representative publication: A Platform Approach to Cleavable Macrocycles for the Controlled Disassembly of Mechanically Caged Molecules.
A. Saady, G. K. Malcolm, M. P. Fitzpatrick, N. Pairault, G. J. Tizzard, S. Mohammed, A. Tavassoli, S. M. Goldup, Angew. Chem. Int. Ed., 2024, 63, e202400344.
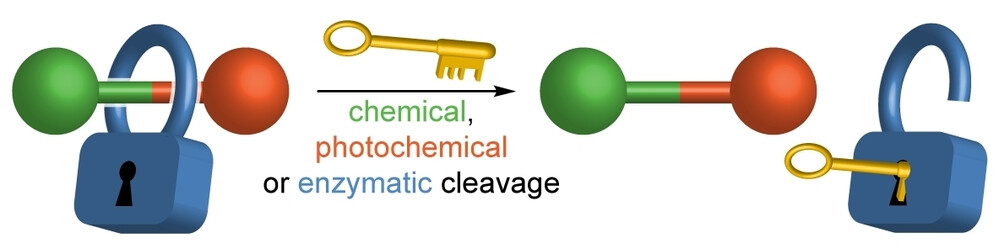
Research in the Goldup Group focuses on the effect of the mechanical bond in catenanes and rotaxanes on the properties and hence applications of these unusual molecules. To achieve this, we have developed some of the most efficient mechanical bond-forming reactions (ACIE 2011; Chem Sci 2015; JACS 2018) and used this methodology to produce mechanically stabilised organometallic species (JACS 2013, Chem. Sci. 2020), interlocked catalysts (ACIE 2015, ACIE 2022), sensors (ACIE 2018; ACIE 2018), ligands to augment the properties of transition metal ions (JACS 2019; ACIE 2021), luminophores (Chem. Sci. 2021, ACIE 2021), synthetic oligonucleotides (JACS 2020) and mechanically caged functional molecules (ACIE 2024 – see selected paper above). We have also used our synthetic methods to address the selective synthesis of structurally challenging catenanes and rotaxanes, in particular structures that display mechanical stereochemistry (JACS 2014; ACIE 2018, Nat. Chem. 2022, Chem 2023 Chem 2019, JACS 2022, Nat. Chem. 2022, Nat. Chem. 2023), and alongside this, developed a unified framework for the discussion of molecules that contain mechanical stereogenic units (Acc Chem. Res. 2024). Using our methodology, we demonstrated the first example of enantioselective catalysis with a mechanically chiral ligand (Chem 2020), highlighting the long-term potential of chiral interlocked molecules.
Synthesis Section Research Group Leaders
Areas of interest: Supramolecular Chemistry – Mechanically Interlocked Molecules – Organic Electronics – Photophysics – Molecular Sensing
Representative publication: The Pink Box: Exclusive Homochiral Aromatic Stacking in a Bis-perylene Diimide Macrocycle.
S. E. Penty, M. A. Zwijnenburg, G. R. F. Orton, P. Stachelek, R. Pal, Y. Xie, S. L. Griffin, T. A. Barendt, J. Am. Chem. Soc., 2022, 144, 12290.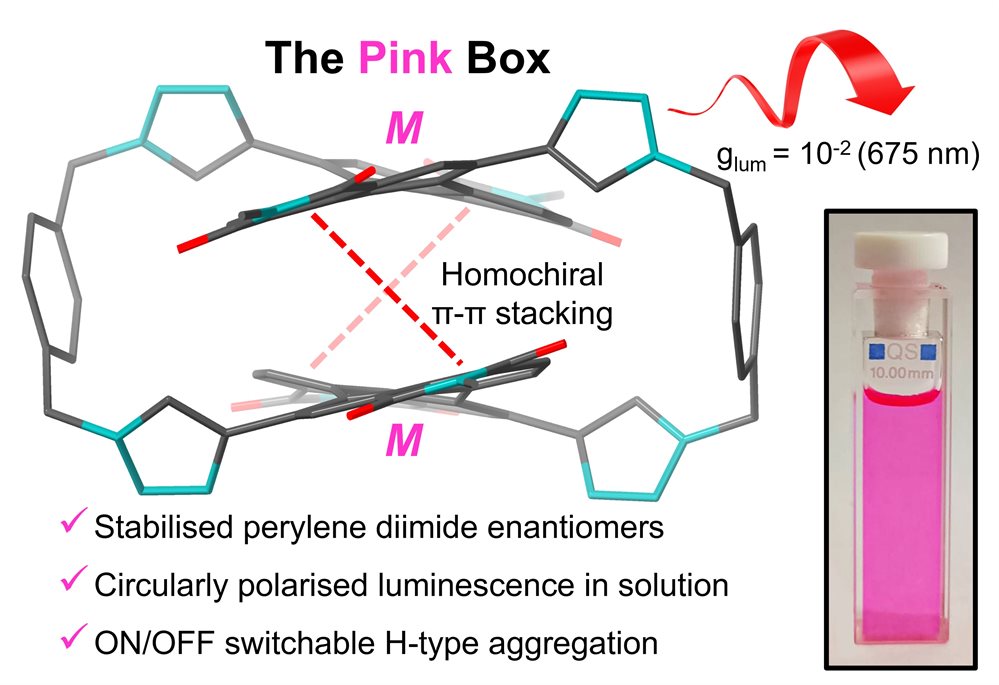
Research in the Barendt group focuses on the synthesis and supramolecular chemistry of organic dyes, to uncover new knowledge of the non-covalent interactions between these aromatic molecules and furthering their applications in (opto)electronic materials and optical sensing. We use archetypal frameworks such as macrocycles and mechanically interlocked molecules to understand how the interactions between dyes and other polycyclic aromatic hydrocarbons can be used to tune their photophysical, electrochemical and chiral properties. The latter is particularly important for the stereoselective synthesis of chiral dyes and the interaction of these organic molecules with circularly polarised light, areas of significant interest to the group. Alongside molecular synthesis and analysis in solution, we also examine the role of supramolecular self-assembly in translating properties to the solid state, including through the fabrication of organic thin film and single crystal materials.
Areas of interest: Supramolecular Chemistry and Self-assembly – Metal-organic Frameworks (MOFs) and Hydrogen-bonded Organic Frameworks (HOFs) – Crystal Engineering –Mechanically-interlocked Molecules: Rotaxanes and Catenanes – Surface self-assembly
Representative publication: Studying manganese carbonyl photochemistry in a permanently porous metal-organic framework.
R.J. Young, M.T. Huxley, L. Wu, J. Hart, J. O'Shea, C.J. Doonan, N.R. Champness, C.J. Sumby, Chem. Sci., 2023, 14, 9409.
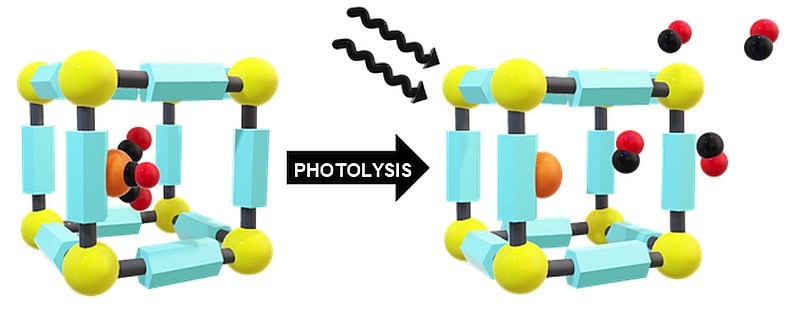
Mechanically interlocked handcuffs provide a strategy to study rylene diimide dimers and to investigate their electronic and magnetic properties.
Areas of interest: Chemical Biology and Bioconjugation Strategies – Diversity-oriented Synthesis – Medicinal Chemistry – Molecular Synthesis and Catalysis
Representative publication: Photoactivable Glycolipid Antigens Generate Stable Conjugates with CD1d for Invariant Natural Killer T Cell Activation.
N. Veerapen, S. S. Kharkwal, P. Jervis, V. Bhowruth, A. K. Besra, S. J. North, S. M. Haslam, A. Dell, J. Hobrath, P. J. Quaid, P. J. Moynihan, L. R. Cox, H. Kharkwal, M. Zauderer, G. S. Besra, S. A. Porcelli, Bioconjugate Chem., 2018, 29, 3161.
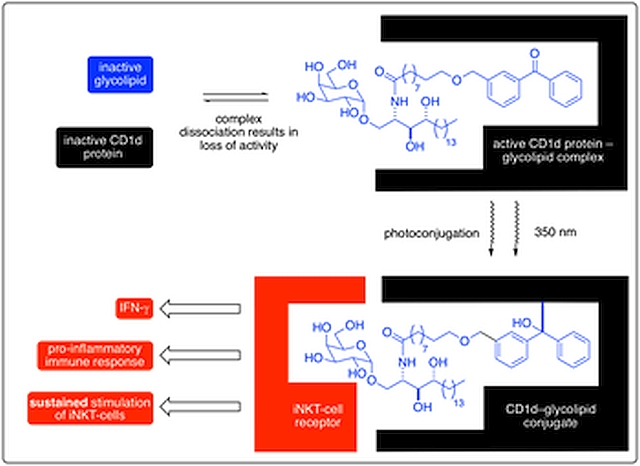
Serial Stimulation of Invariant Natural Killer T Cells with Covalently Stabilized Bispecific T Cell Engagers Generates Anti-Tumor Immunity While Avoiding Anergy. S. S. Kharkwal et al. Cancer Res, 2021, 81, 1788.
A wide range of glycolipids bind to the protein CD1d. Through judicious choice of glycolipid, the resulting complex is capable of activating iNKT cells to elicit an immune response. Whilst careful optimisation of the glycolipid structure has delivered some potent iNKT-cell activators, dissociation of the glycolipid ligand from the CD1d molecule remains a problem for their potential therapeutic application in immunotherapies. In this paper, we describe the first functional CD1d–glycolipid conjugate in which ligand dissociation is now no longer an issue. This bioconjugation technology provides novel tool compounds for studying the mechanism of iNKT-cell biology and opens up a new immunotherapy strategy for future clinical application.
Areas of interest: Catalysis – Organic Synthesis – Transition Metals – Carbenes – Heterocycles – Gold – Ligands – Method Development.
Representative publication: Gold(I)-Catalyzed Synthesis of 3-Sulfenyl Pyrroles and Indoles by a Regioselective Annulation of Alkynyl Thioethers.
P. E. Simm, P. Sekar, J. Richardson, P. W. Davies, ACS Catal., 2021, 11, 6357.
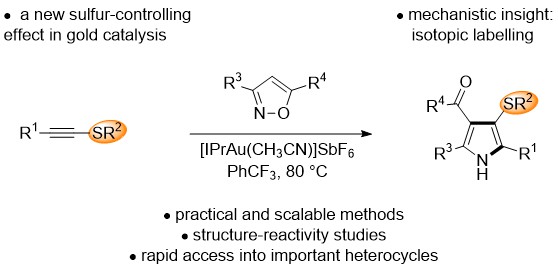
My group is interested in catalysis and organic synthesis, from reaction discovery and development to catalyst design, and the use of catalysis to access novel functional molecules. In reaction discovery, we are introducing new transition metal catalysed transformations to access more efficient and sustainable chemical methods. Our synthetic work is combined with mechanistic studies and computational collaborations to gain further insight into new reactivity. We are particularly interested in complexity-increasing processes that make multiple bonds in one step. Major themes in the group include: (i) using gold chemistry to access carbene-like reactivity from simple alkynes, providing access to powerful methods for bond formation without the hazards and synthetic waste that are normally associated with carbene precursors; (ii) new nitrenoid chemistry for faster access into nitrogen heterocycles; (iii) exploring new controlling effects in gold catalysis.
In catalyst development, we are exploring new multifunctional and responsive ligand motifs to achieve greater control over catalyst reactivity and selectivity, including asymmetric transformations and chemistry in water.
We are also exploring how catalysis can enable fields such as drug discovery and organic electronics. Catalysis tools are used to provide modular approaches into desirable targets that allow faster and more efficient synthesis and enable structure-function/activity studies, and to explore uncharted regions of chemical space and molecular function
Areas of interest: Rotaxanes – Polymers – Nanoparticles – Supramolecular Chemistry – Molecular Machines
Representative publication: Triggered Polymersome Fusion.
S. D. P. Fielden, M. J. Derry, A. J. Miller, P. D. Topham, R. K. O'Reilly, J. Am. Chem. Soc., 2023, 145, 5824.
Steve's research interests are centred around supramolecular chemistry and polymer nanotechnology. His current research focusses on the controlled fusion of nanoparticles and the synthesis/application of interlocked molecules. Steve applies chemical reaction networks and polymerisations to generate nanotechnology that resides and operates in nonequilibrium states. This allows for control over dynamic and responsive behaviour.
Areas of interest: Synthetic Organic Chemistry – Free Radicals – Photochemistry – Natural Product Synthesis – Organosulfur and Organoselenium Chemistry – Peri-interactions – Medicinal Chemistry
Representative publication: Carbamoyl radical-mediated synthesis and semipinacol rearrangement of β-lactam diols.
M. Betou, L. Male, J. W. Steed, R. S. Grainger, Chem. Eur. J., 2014, 20, 6505.
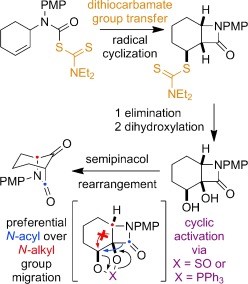
This paper reports a conceptually novel entry into bridged bicyclic lactams through ring expansion of fused beta-lactam diols. The core structures that can be prepared through this methodology are represented in a range of biologically important natural and non-natural products, and are ripe for further functionalization as rigid three-dimensional scaffolds for medicinal chemistry applications. The work builds on our group’s free-radical cyclisation methodology to access functionalized, cis-fused bicyclic lactams. The use of cyclic phosphoranes to rearrange diols has little precedent and through this work is shown to be potentially a more general alternative to the rearrangement of epoxides.
Areas of interest: Main-group Chemistry – Sustainable Synthesis – Small-molecule Activation – Catalysis – Photochemistry – Phosphorus
Representative publication: Synthesis and Reactivity of the [NCCCO]− Cyanoketenate Anion.
T. Wang, Z. Guo, L. E. English, D. W. Stephan, A. R. Jupp, M. Xu, Angew. Chem. Int. Ed., 2024, 63, e202402728.
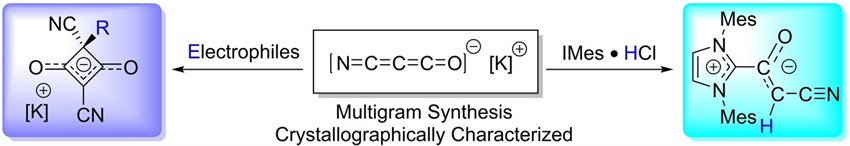
Frustrated Lewis Pairs (FLPs) are combinations of Lewis acids and bases that are sterically or electronically prevented from forming stable Lewis adducts. The unquenched acid and base sites, typified by bulky boranes and phosphines, respectively, can subsequently be exploited for small-molecule activation. We showed that light can be used to promote single-electron transfer from the Lewis base to the Lewis acid, forming a radical ion pair. This was characterised using UV-Vis spectroscopy (Figure a, above), EPR spectroscopy (Figure b) and transient absorption spectroscopy (Figure c). We were able to use the findings from this spectroscopic study to further interrogate the mechanism of the activation of dihydrogen (H2) and tin hydrides (R3SnH) by a range of FLPs.

Royal Society University Research Fellow
Associate Professor in Supramolecular Chemistry
School of Chemistry
- Email
- j.e.m.lewis@bham.ac.uk
Areas of interest: Supramolecular chemistry – Metal-organic self-assembly – Coordination cages – Mechanically interlocked molecules.
Representative publication: Diastereoselective self-assembly of low-symmetry PdnL2n nanocages through coordination-sphere engineering.
P. Molinska, A. Tarzia, L. Male, K. E. Jelfs, J. E. M. Lewis, Angew. Chem. Int. Ed., 2023, 62, e202315451
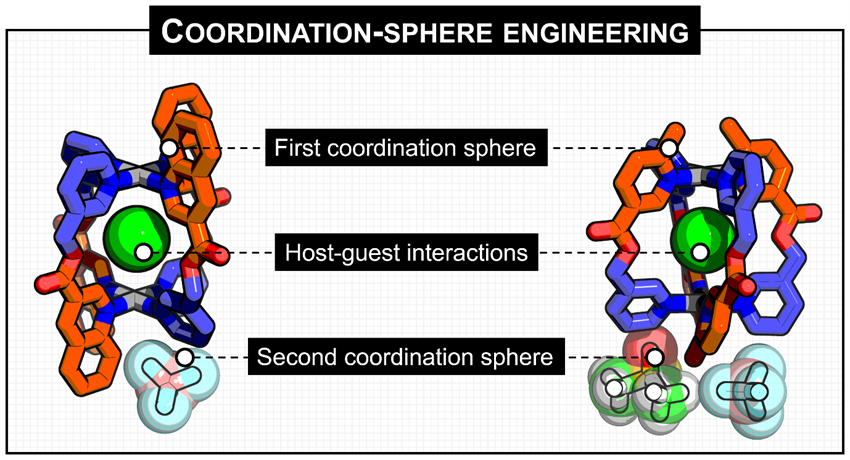
Research in the Lewis Group is centered around synthetic supramolecular chemistry. We have particular research themes in the development and investigation of capsule-type molecular hosts, including coordination cages, and the incorporation of mechanically interlocked molecules (MIMs) into materials. In the area of coordination cages, we have developed principles to access information-rich, low-symmetry cages through orientation-selective assembly of low-symmetry ligands, including through geometric design (Chem. Sci. 2020, 11, 677; Chem. Eur. J. 2021, 27, 4454; ACIE 2021, 60, 20879), covalent tethering (ACIE 2022, 61, e202212392) and coordination-sphere engineering (ACIE 2023, 62, e202315451) strategies. In the field of MIMs we have incorporated interlocked components into discrete (Chem. Commun. 2020, 56, 10453) and polymeric (Org. Biomol. Chem. 2019, 17, 2442) coordination assemblies, and investigated the impact of the mechanical bond on functional group properties (Chem. Sci. 2022, 13, 11368) and the delivery and stimuli-responsive release of cytotoxic agents (ACIE 2021, 60, 10928).
Areas of interest: Metal-organic Framework – Cages – Reticular Materials – Supramolecular Chemistry – Crystallography
Dr Georgia Orton's research interests span i) the synthesis of framework materials and air-sensitive molecules, ii) host-guest chemistry, and iii) understanding structure and reactivity via crystallography and spectroelectrochemistry.
Georgia completed her MChem (Europe) at the University of Sheffield in 2015, including a year at The University of Bordeaux. Following this, she joined the Hogarth group at King’s College London for a PhD focusing on producing biomimics of the iron-only hydrogenase enzyme as electrocatalysts for hydrogen oxidation. In 2019, Georgia joined the Champness group, initially in Nottingham and later in Birmingham, to work on metal-organic frameworks for mechanistic studies of reactions within frameworks.
In October 2023 Georgia took up a Leverhulme Trust Early Career Fellowship. Her current research focuses on reactivity of reticular materials, and the design and synthesis of unusual metal-organic molecules and materials.
Areas of interest: Supramolecular Chemistry – Molecular Recognition Toolbox – Metallosupramolecular architectures – Host-guest Chemistry – Sensing
Representative publication: H-Bond donor parameters for cations.
S. J. Pike, E. Lavagnini, L. M. Varley, J. L. Cook, C. A. Hunter, Chem. Sci., 2019, 10, 5943.
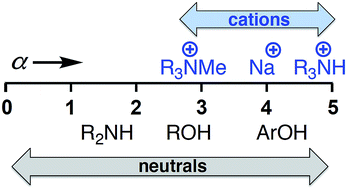
In this work, we have determined self-consistent H-bond donor parameters (α) for a series of organic and inorganic cations, including guanidinium, primary, tertiary and quaternary ammonium, lithium, sodium and potassium. The transferability of α parameters for cations between different solvents and different H-bond acceptor partners allows for the reliable prediction of cation recognition properties in a range of different environments. These new H-donor parameters for cations will be useful in the development of our understanding and prediction of the behaviour of charged species in organic solution with applications in the design of new supramolecular systems.
Research Collaborations
Section members are actively engaged with both industrial and academic partners within the UK and overseas as well as in collaborative projects across the University of Birmingham (School of Biosciences, School of Chemical Engineering and the College of Medical and Dental Sciences). This includes cross-disciplinary areas of research activity covered by the School of Chemistry's research themes, in particular Chemical Biology and Drug Discovery and Chemistry for Healthcare Technologies.
Wider engagement
Research carried out in the Synthesis Section plays a crucial role in maintaining and improving our standard of life. Members of the Section work closely with the Royal Society of Chemistry, the Society of Chemical Industry and the Royal Society as committee members and Fellows, to enable effective knowledge transfer across academia and industry and to enhance public awareness of this central area of Chemistry.
Contact
Enquiries about specific aspects of their respective research areas should be addressed to individual research group leaders. For more general enquiries about working with the Synthesis Section, please contact ProfessorSteve Goldup (Section Lead). Information on various postgraduate (PhD and Masters) degree opportunities can be found on our postgraduate opportunities page.