Professor Steve Goldup
- Professor of Chemistry
- School of Chemistry
- Research Group webpage
- Twitter: @golduplab
Areas of interest: Rotaxanes and Catenanes – Stereochemistry – Prodrugs – Coordination Chemistry – Catalysis
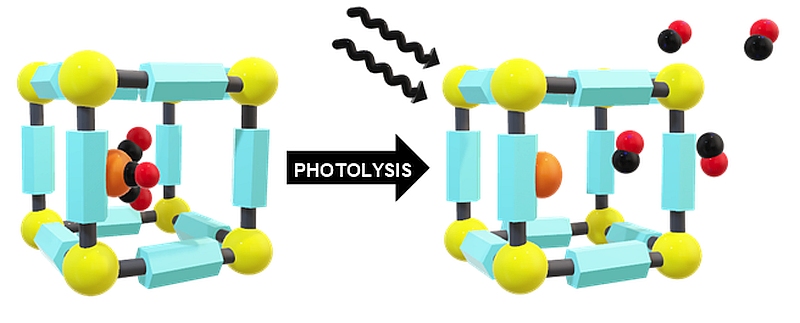
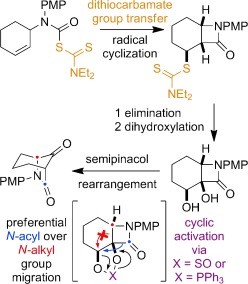
Representative publication: A Platform Approach to Cleavable Macrocycles for the Controlled Disassembly of Mechanically Caged Molecules.
A. Saady, G. K. Malcolm, M. P. Fitzpatrick, N. Pairault, G. J. Tizzard, S. Mohammed, A. Tavassoli, S. M. Goldup, Angew. Chem. Int. Ed., 2024, 63, e202400344.
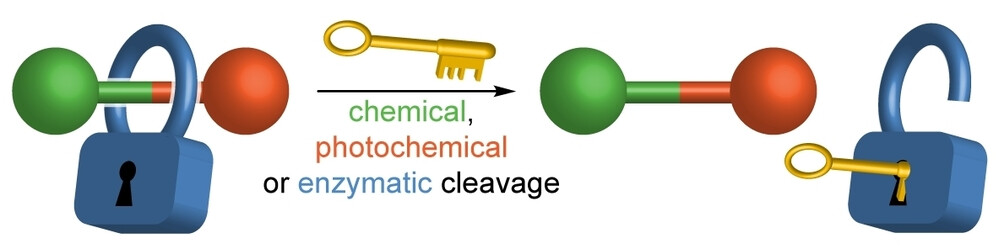
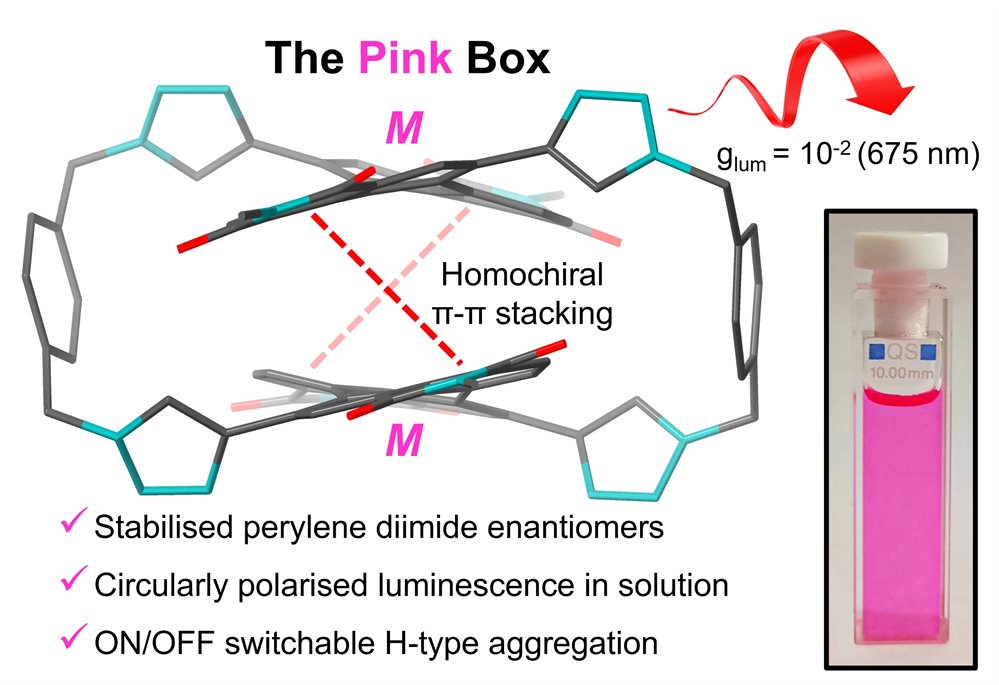
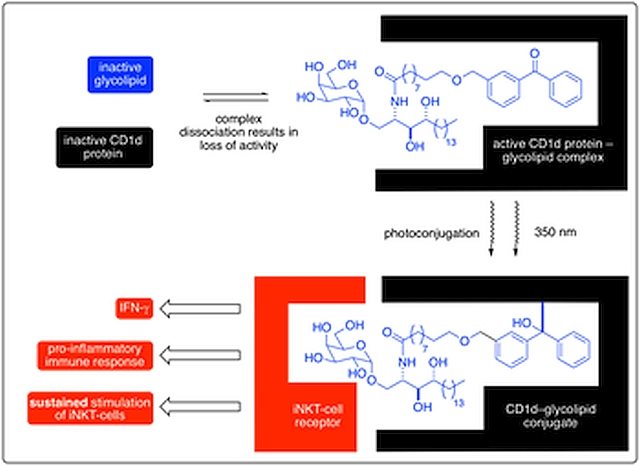
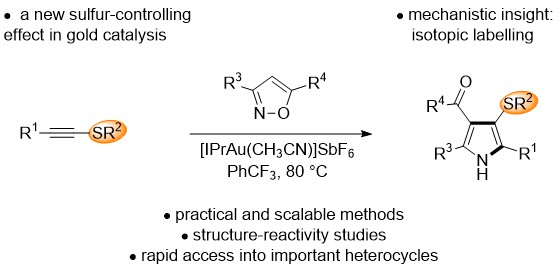
Research in the Goldup Group focuses on the effect of the mechanical bond in catenanes and rotaxanes on the properties and hence applications of these unusual molecules. To achieve this, we have developed some of the most efficient mechanical bond-forming reactions (ACIE 2011; Chem Sci 2015; JACS 2018) and used this methodology to produce mechanically stabilised organometallic species (JACS 2013, Chem. Sci. 2020), interlocked catalysts (ACIE 2015, ACIE 2022), sensors (ACIE 2018; ACIE 2018), ligands to augment the properties of transition metal ions (JACS 2019; ACIE 2021), luminophores (Chem. Sci. 2021, ACIE 2021), synthetic oligonucleotides (JACS 2020) and mechanically caged functional molecules (ACIE 2024 – see selected paper above). We have also used our synthetic methods to address the selective synthesis of structurally challenging catenanes and rotaxanes, in particular structures that display mechanical stereochemistry (JACS 2014; ACIE 2018, Nat. Chem. 2022, Chem 2023 Chem 2019, JACS 2022, Nat. Chem. 2022, Nat. Chem. 2023), and alongside this, developed a unified framework for the discussion of molecules that contain mechanical stereogenic units (Acc Chem. Res. 2024). Using our methodology, we demonstrated the first example of enantioselective catalysis with a mechanically chiral ligand (Chem 2020), highlighting the long-term potential of chiral interlocked molecules.