ROSSINI 2 is a phase III, multi-arm, multi-stage (MAMS), pragmatic, blinded (patient and outcome assesor), multicentre, randomised controlled trial, with an internal pilot, to evaluate the use of several in-theatre interventions, used alone or in combination, to reduce the rates of SSIs in patients undergoing surgery with an abdominal incision. A non-factorial superiority design with allocation to various combinations of the trial interventions to be used during the same operation.
SSI prevention lends itself well to an adaptive or multi-arm, multi-stage (MAMS) trial design, because the primary outcome result is, by definition, available 30 days after the intraoperative intervention is applied. Interim analyses can exploit this short timeline to create an efficient trial that will evaluate several interventions (both individually and in combination) under a single umbrella structure. This decreases both the time and cost investment necessary to simultaneously determine if several non-bundled interventions are effective. Determining small incremental benefits will be slow and difficult to achieve. This trial design allows exploration of interactions between interventions, which each have a different biological mechanism in this multifactorial process.
The trial would be the first of its kind in a surgical setting. In addition to generating new knowledge in our primary research area, by utilising this advanced design in the context of our relatively simple primary endpoint of SSI, it will also pave the way for future efficient and rapid trials in other aspects of surgical care.
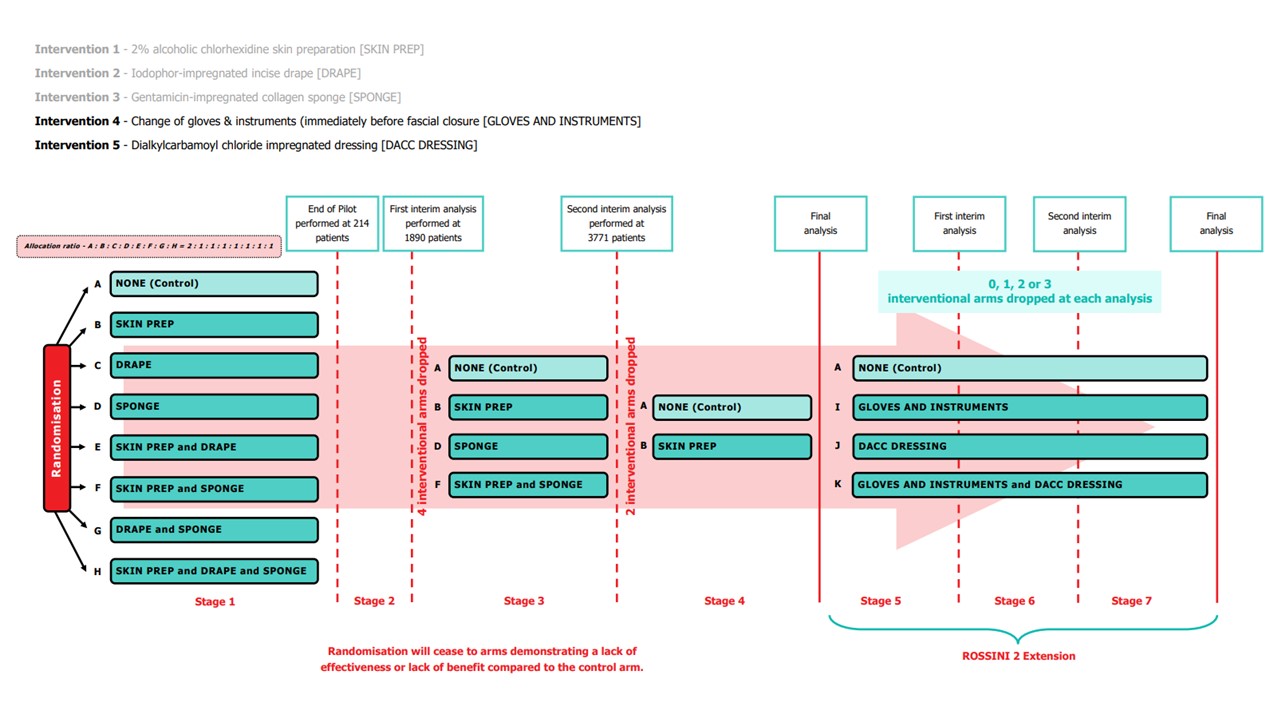
The interventions chosen in ROSSINI 2 impact different phases of perioperative case and as such can be used either in isolation or in conjunction with each other and although there may potentially be interaction between the interventions (positive or negative) they appear to be mechanistically disparate.
The current interventions are:
-
Change of Gloves and Instruments immediately before fascial closure vs standard practice
-
Dialkylcarbamoyl chloride (DACC) impregnated dressing
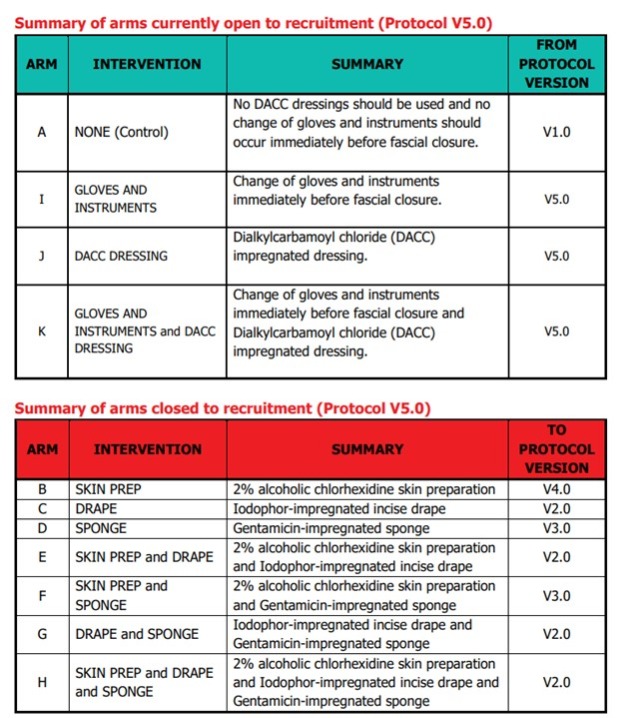
Please note: In January 2022, following the first interim analysis, arms including intervention 'Iodophor-impregnated incise drape' (arms C, E, G and H) were closed to recruitment. In January 2023, following the second interim analysis, arms including intervention 'Gentamicin-impregnated sponge (arms D and F) were closed to recruitment.
At least 60 NHS hospitals in the UK will participant in ROSSINI 2.
Approximately 4269 patients will be required to detect a 5% absolute risk reduction in the interventions arm(s) (15% to 10%; 33% relative reduction) with 85% power.
Pilot Stage Summary of Completion (Stage 1)
The Independent Trial Steering Committee (TSC) and Data Monitoring Committee (DMC) held a joint meeting on 30th September 2019 to review the internal pilot stage objectives and results. They were satisfied with the progress against the predefined progression criteria and recommended that ROSSINI 2 continue to the main phase of the trial without the need for any major modifications. The NIHR also reviewed trial progress and reports from the oversight committees; they confirmed progression to the main phase in October 2019. The table below details the success of the internal pilot stage:
 Stage 2 Summary of Completion (First Interim Analysis)
Stage 2 Summary of Completion (First Interim Analysis)
It was agreed in an extraordinary meeting of the Trial Management Group (TMG), TSC and DMC on October 5th 2021 (and subsequently by the Funder) that it would be reasonable to bring forward the first interim analysis i.e. sooner than 2357 patients as originally planned. This decision was based on observing a slightly higher than expected overall event rate.
On December 15th 2021, the results of the first interim analysis were presented and reviewed by the independent DMC. They made the recommendation to drop the intervention arms containing Intervention 2, Iodophor-impregnated incise DRAPE. The DMC recommendations were discussed by the ROSSINI 2 TMG, independent TSC, Sponsor and Funder at a meeting on January 7th 2022. Following this meeting, it was agreed that the following arms containing Intervention 2, Iodophor-impregnated incise DRAPE, would close to recruitment:
All other aspects such as the primary objective, trial design, trial setting, outcome measures and key eligibility criteria remain unchanged.
Stage 3 Summary of Completion (Second Interim Analysis)
A total of 3771 participants had been randomised – of whom 952 were randomised to arms that had been dropped after the last DMC, leaving 2819 randomised participants in the trial; 1108 to the control arm and approximately 570 to each of the intervention arms. Of these, 973 controls and approximately 500 in each intervention have primary outcome data available for analysis at this interim analysis giving a total of 2475 people for analysis.
On January 25th 2023, the results of the second interim analysis were presented and reviewed by the independent DMC. They made the recommendation to drop the intervention arms containing Intervention 3, Gentamicin-impregnated SPONGE. The DMC recommendations were discussed by the independent TSC, the ROSSINI 2 TMG and Sponsor at a meeting on January 31st 2023. Following the meeting, it was agreed that the following arms containing Intervention 3, Gentamicin-impregnated SPONGE, would close to recruitment:
-
Arm D SPONGE
-
Arm F SKIN PREP & SPONGE
All other aspects such as the primary objective, trial design, trial setting, outcome measures and key eligibility criteria remain unchanged.
Stage 4 Summary of Completion (Final Analysis of the original ROSSINI 2 trial)
Pending Stage 4 Summary of completion/final analysis due early 2025.
Stage 5 (ROSSINI 2 Extension)
The health technologies that will be assessed versus the control arm (standard care):