
Dr Daniel Fulton
Associate Professor in Glial Biology
Daniel Fulton is an Associate Professor in Glial Biology based in the Neuroscience and Ophthalmology group.


The Oligodendrocyte Research Group studies the development and repair of myelin, the insulating material that ensures efficient nerve impulse conduction and supports the heath of the axon. Damage to myelin through traumatic injury and disease disturbs the coordinated flow of information throughout the CNS, and compromises the health of the underlying axons, leading to impairments in cognition and movement.
The aim of our research is to develop a better understanding of the cellular and molecular mechanisms controlling myelin formation, injury, and repair, and identify novel therapeutic targets capable of protecting CNS myelin and promoting its repair. The group is pursuing these goals with a range of molecular, biochemical and cellular imaging techniques in oligodendrocyte cell lines, primary and stem cell derived oligodendrocytes, and organotypic brain slice cultures.
As a part of Birmingham’s Department of Inflammation and Ageing, and its Neuroscience and Ophthalmology division, the group enjoys close interactions with researchers focused on complementary subjects including neuro-protection and axon regeneration (Professor Zubair Ahmed), and inflammatory demyelination / Multiple Sclerosis (Professor Michael Douglas, Professor Zubair Ahmed).
Hypoxic-ischemic events in the late stages of pregnancy threaten immature oligodendrocytes (OL) and developmental myelination through mechanisms involving excitotoxicity, oxidative stress and inflammation (reviewed here). The oligodendrocyte group are using primary OL cultures and organotypic brain slice models to identify novel pathways capable of protecting OL under these conditions. Projects in this area include work on AMPA-type glutamate receptors (Fannon et al.) and cannabinoid pathways (link to Maria section). For this work we are using two brain slice injury models: (1) ischemic-excitotoxicity, where slices are deprived of glucose and stimulated with excess glutamate (Figure 1); and (2) hypoxic-ischemic injury, where slices will be deprived of glucose in a modular incubator system gassed with 2.5% oxygen.
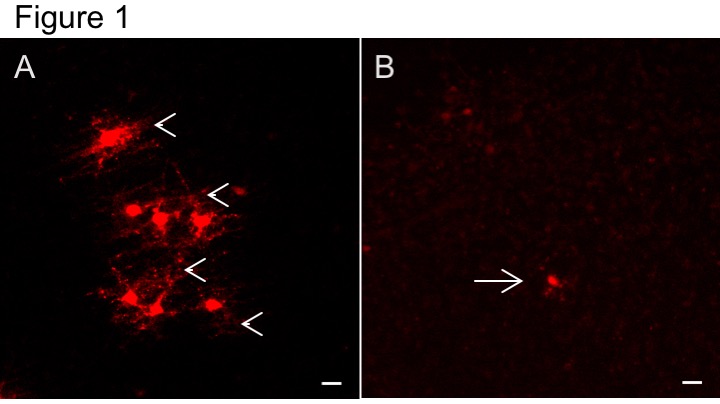
Figure 1. Ischemic-excitotoxic injury in forebrain slice cultures. Forebrain slices were deprived of glucose for 2h before exposure to glutamate (3.5 mM) for 35 min. A. Viable myelinating transgenic dsRED+ OL are seen in the cortex of control non-injured slices 6 days after treatment. Note parallel aligned dsRED OL processes indicative of myelin sheaths (arrowheads). B. 6 days after ischemic-excitotoxic injury dystophic OL are scattered through the tissue (arrow), but no myelinating dsRED+ OL are observed. Scale bars 20 µm.
Myelin formation involves dynamic physical interactions between oligodendrocytes and their axonal targets that are influenced by electrical activity and neurotransmitter release from the target axon (Fannon et al., and other studies). In vivo imaging of larval zebrafish and Xenopus have illuminated the physical events driving the initial stages of myelination (Reviewed by Rassul et al.), and uncovered an important role for neuronal activity in these events. How these findings from lower vertebrates relate to the situation within intact mammalian CNS tissue remains unclear.
To study these questions we are imaging OL maturation in cortical brain slice cultures using transgenic OL reporter mice such as the PLPdsRED line (Fig 2, dsRED time-lapse). New approaches developed in the Fulton group provide a means to image the early stages of myelination as immature OL begin to elaborate their first myelin segments. Daniel recently gave a talk on these methods at the Aurox Conference on Microscopy 2020. You can watch this talk on YouTube.
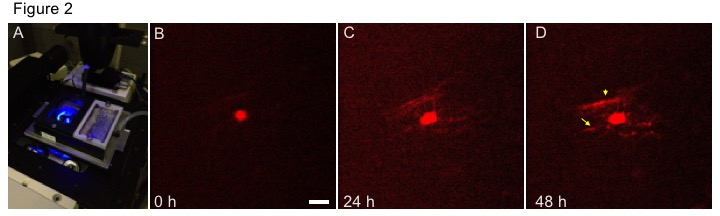
Figure 2. Live imaging of OL myelination in forebrain slice cultures. A. Forebrain slices the PLP-dsRED OL reporter line are imaged on a spinning disc confocal microscope. During imaging brain slice cultures are maintained at 37.C / 5%Co2 in an environmental chamber B-D. Imaging over a period of 48 hours reveals the initial stages of OL myelination. Over 3 imaging sessions the PLP-dsRED transgene is upregulated revealing OL processes as they begin to ensheath axons and develop into immature myelin sheaths (yellow arrows). Scale bars 20 µm.
The myelin sheath contains a mixture of specialized ‘myelin’ proteins and lipids that are compacted down to form a tight insulating material. Inflammatory / excitotoxic injury to OL myelin underlies a range of neurological diseases (most notably multiple sclerosis), yet how these injuries evolve within the myelin-molecular environment remains under-investigated. Indeed, Single-molecule imaging (SMI) methods can reveal these events since they allow individual molecules to be tracked and their motions analysed quantitatively (Link to Rassul et al.). Using SMI to track the motion of individual MBP molecules we have begun to analyse the behaviour of this key myelin protein during OL differentiation and myelin injury (Figure 3). Observations of MBP motion during myelin injury promises to reveal new insights into the molecular interactions that maintain the molecular structure of myelin.
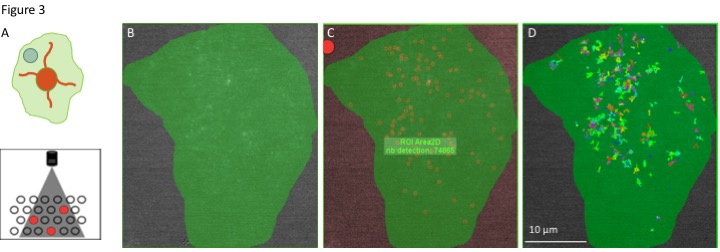
Figure 3. Single Molecule Imaging of MBP in myelin sheets. A. Upper panel: Cartoon depicting a myelinating OL. Green area depicts the myelin sheet, a flattened myelin structure formed by myelinating OL in vitro. Teal circle indicates region of interest for imaging. Lower panel: SMI methods allow the behaviour of a sub-population (red circles) of molecules to be observed within the whole population. B. Imaging of a myelin sheet expressing denra2-MBP (grey signal). Photoconvertion of dendra2-MBP by illumination of the sample with 405nm light prior to imaging produces a sparse signal suitable for SMI analysis. C. Detection of single dendra2-MBP molecules by ICY software. D. Tracks showing the motion of individual MBP molecules shown in C. Tracks were resolved from time-lapse imaging experiments (1 frame / 30 ms, 2500 frames, 160x 1.4 N.A objective). Track data can be used to calculate motion data such as diffusion and confinement ratios.
This project, funded by Ono Pharmaceutical Co. Ltd., is investigating the role of bioactive lipids in the pro-differentiation cross-talk between microglia and oligodendrocyte precursor cells (OPC).
AMIGO3 is a member of the leucine rich repeat superfamily of proteins (LRR) which has been shown to be expressed in oligodendrocytes, with raised levels observed in demyelinating diseases and CNS trauma. LINGO1, another member of the LRR with an analogous structure to AMIGO3, has been demonstrated to inhibit oligodendrocyte precursor cells (OPC) from maturing and producing myelin both in vitro and in vivo in murine demyelination models. Our previous studies have demonstrated that LINGO1 and AMIGO3 have a shared mechanism of action in neurons and it is hypothesised that AMIGO3 is able to similarly inhibit OPC differentiation in disease. We are exploring these issues in cell cultures, organotypic brain slice cultures and a mouse model for inflammatory demyelination (experimental autoimmune encephalomyelitis). Subsequent analysis of maturation, myelination and clinical disease scores will help determine the relevance of AMIGO3 signaling to myelin repair therapies.
ES cells may provide a useful source of myelinating oligodendrocytes for achieving myelin repair in the injured CNS. Neuronal activity and membrane depolarisation enhance myelination, and promote the differentiation of ES cells, yet the impact of depolarisation on the generation of oligodendrocytes from ES is unknown, as are the molecules driving depolarisation-induced ES differentiation.
STEM-ZAP, an H2020 funded Marie Sklowdowska-Curie project, is using optogenetic stimulation, high-throughput screening and transcriptomic analyses to identify novel signalling pathways involved in the generation of myelinating oligodendrocytes.
Manukjan N, Ahmed Z, Fulton D, Blankesteijn WM, Foulquier S. A Systematic Review of WNT Signaling in Endothelial Cell Oligodendrocyte Interactions: Potential Relevance to Cerebral Small Vessel Disease. Cells. 2020 Jun;9(6):1545.
Ahmed Z, Fulton D, Douglas MR. Opicinumab: is it a potential treatment for multiple sclerosis?. Annals of Translational Medicine. 2020 Jul;8(14).
Toth E, Rassul SM, Berry M, Fulton D. A morphological analysis of activity-dependent myelination and myelin injury in transitional oligodendrocytes. bioRxiv. 2019 Jan 1:750083.
Ceprian M, Fulton D. Glial cell AMPA receptors in nervous system health, injury and disease. International journal of molecular sciences. 2019 Jan;20(10):2450.
Toth E, Rassul SM, Berry M, Fulton D. (2019) A morphological analysis of activity-dependent myelination and myelin injury in transitional oligodendrocytes. bioRxiv. 2019 Jan 1:750083. https://doi.org/10.1101/750083
Ceprian M and Daniel Fulton. (2019) Glial Cell AMPA Receptors in Nervous System Health, Injury and Disease. International journal of molecular sciences 20.10 (2019): 2450. https://doi.org/10.3390/ijms20102450
Begum G, Otsu M, Ahmed U, Ahmed Z, Stevens A, Fulton D. (2018) NF-Y-dependent regulation of glutamate receptor 4 expression and cell survival in cells of the oligodendrocyte lineage. Glia. 66: 1896-1914. DOI: 10.1002/glia.23446.
Berry M, Ahmed Z, Morgan-Warren P, Fulton D and Logan A (2016) Prospects for mTOR-mediated functional repair after central nervous system trauma. Neurobiology of Disease 85:99-110
Rassul SM, Neely RK and Fulton D (2015) Live-imaging in the CNS: Insights on oligodendrocytes, myelination, and their responses to inflammation. Neuropharmacology [Epub ahead of print]
Fanning J, Tarmier W and Fulton D (2015) Neuronal activity and AMPA-type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia 63(6):1021-35
Leoni G, Rattray M, Fulton D, Rivera A and Butt AM (2014) Immunoablation of cells expressing the NG2 chondroitin sulphate proteoglycan. Journal of Anatomy 224(2):216-27
Fulton D, Paez P, Spreur V, Handley V, Colwell CS, Campagnoni A & Fisher R (2011). Developmental activation of the proteolipid protein (PLP) promoter transgene in neuronal and oligodendroglial cells of the neostriatum in mice. Developmental Neuroscience. 33: 170-184. *Selected for Editor's Choice*.
Paez PM, Fulton D, Spreuer V, Handley V & Campagnoni AT (2011). Modulation of Canonical Transient Receptor Potential Channel 1 in the Proliferation of Oligodendrocyte Precursor Cells by the Golli Products of the Myelin Basic Protein Gene. Journal of Neuroscience. 31, 3625-3637.
Fulton D, Paez PM, Fisher R, Handley V, Colwell CS & Campagnoni AT (2010). Regulation of L-type Ca++Currents and process morphology in white matter oligodendrocyte precursor cells by golli-myelin proteins. Glia. 58, 1292-1303.
Paez PM, Fulton D, Spreuer V, Handley V & Campagnoni AT (2009). Multiple kinase pathways regulate voltage-dependent Ca++ influx & migration in oligodendrocyte precursor cells. Journal of Neuroscience. 30, 6422-643.
Paez PM, Fulton DJ, Spreuer V, Handley V, Campagnoni CW & Campagnoni AT (2009). Golli Myelin Basic Proteins Regulate Oligodendroglial Progenitor Cell Migration through Voltage-Gated Ca2+ Influx. Journal of Neuroscience. 29, 6663-6676.
Fulton D1, Paez PM1 & Campagnoni AT. (2009) The multiple roles of myelin protein genes during the development of the oligodendrocyte. ASN NEURO. 1(1): doi:10.1042/AN20090051. 1Equal author contribution.
Paez PM, Fulton DJ, Spreuer V, Handley V, Campagnoni CW & Campagnoni AT (2009). Regulation of store-operated and voltage-operated calcium channels in the proliferation and death of oligodendrocyte precursor cells. ASN Neuro. Doi:10.1042/AN20090003.
Fulton D, Kemenes I, Andrew RJ & Benjamin PR (2008). Time-window for sensitivity to Cooling Distinguishes the Effects of Hypothermia and Protein Synthesis Inhibition on the Consolidation of Long-Term Memory. Neurobiology of Learning and Memory. 90, 651-654.
Fulton D, Condro MC, Pearce K & Glanzman DL (2008). The potential role of postsynaptic phospholipase C activity in synaptic facilitation and behavioral sensitization in Aplysia. Journal of Neurophysiology. 100, 108-116
Fulton D (2006) Reconsolidation: does the past linger on. Journal of Neuroscience, 26 10935-10936.
Fulton D, Kemenes I, Andrew RJ & Benjamin PR. (2005). A single time-window for protein-synthesis-dependent long-term memory formation after one-trial appetitive conditioning. European Journal of Neuroscience, 21, 1347-1358.
Daniel’s doctoral work (Benjamin Lab, University of Sussex, UK) and early postdoctoral research (Glanzman Lab, University of California, Los Angeles, link to Glanzman Lab) investigated the cellular and molecular mechanisms of learning and memory. He then transferred his electrophysiological expertise to the study of oligodendrocyte development in the laboratory of Anthony Campagnoni (University of California, Los Angeles). During this time he determined the influence of novel myelin proteins on the regulation of voltage-gated calcium channels in developing oligodendrocytes, and investigated the expression of the myelin proteolipid protein (PLP) gene in striatal projection neurons in the developing CNS.
Daniel moved back to the UK in 2011 to take up an independent fellowship at the University of Warwick (Science City Research Alliance Senior Fellowship) where he initiated work investigating the role of neuronal activity on oligodendrocyte development and myelination. In 2013 Daniel secured a Birmingham Fellowship which has enabled him to establish the oligodendrocyte and myelin biology group within Birmingham’s Neurotrauma Research Group.

Mo was a student within Birmingham’s Physical Sciences of Imaging in the Biomedical Sciences (PSIBS) Doctoral Training Centre. Mo undertook his undergraduate degree in Neuroscience with Psychology, at The University of Aberdeen, where his dissertation project investigated the role of Epac signalling in CNS regeneration. Mo’s doctoral work involved the development of single molecule imaging methods for the study of myelin proteins, and their responses to myelin injury. He successfully completed his PhD in December 2018 and is now employed as a researcher on a new project funded by Ono Pharmaceutical that is exploring the role of Eicosanoid pathways in oligodendrocyte differentiation.

Narek is doctoral student jointly funded through a collaboration between the Universities of Birmingham and Maastricht. His research is focussed on cross-talk between endothelial cells and oligodendrocyte precursor cells, its role in driving oligodendrocyte differentiation, and its regulation by hypoxic injury.

Maria visited the Oligodendrocyte group in late 2017 to undertake a 3-month research project associated with her PhD studies. This PhD work, which was jointly undertaken between Medical School at the Universidad Complutense de Madrid and the Institute of Children and Adolescents at the Hospital Clinico San Carlos (Madrid, Spain), characterised the neuro- and myelin-protective effects of CBD in rodent models of neonatal hypoxic-ischemic (HI) brain injury. Her visit to Birmingham complemented this in vivo work by providing the opportunity to perform pilot experiments exploring oligo-toxic and oligo-protective actions of CBD in cerebellar slice cultures.

Ghazala obtained her PhD in Neuroendocrinology from the University of Manchester (White lab, UK) investigating epigenetic regulation of the energy pathway in the hypothalamus. During this time she developed her understanding of chromosomal and transcriptional regulation. Ghazala undertook postdoctoral training in the Oligodendrocyte research group where she investigated the transcriptional regulation of AMPA receptors in oligodendrocytes, and their regulation during excitotoxic injury. Her work also identified the nuclear factor Y complex as a novel regulator of oligodendrocyte survival. Ghazala's work has been published in Glia https://doi.org/10.1002/glia.23446. Ghazala is now a Senior Research Manager at Mirna Diagnostics ltd.

Masahiro undertook a Marie Skłodowska-Curie individual Fellowship in the Oligodendrocyte research group. His research on the STEM-ZAP (UoB) project developed novel methods for the generation of early NG2-glia from embryonic stem cells. Masa is now a R&D Manager at Braizon Therapeutics Inc.

Simon was an MRC funded doctoral student whose research investigated the regulation of leucine rich proteins, primarily AMIGO3, during oligodendrocyte differentiation and myelination. Simon successfully completed his PhD in December 2018.

Majed was a doctoral student funded through the Saudi Arabian Cultural Mission. Majed's research used primary CNS cell culture models to investigate the role of leucine rich proteins, primarily AMIGO3, in regulating oligodendrocyte differentiation and myelination.
European Neuro Convention
Date: 9-10 March 2021
Birmingham, UK. NEC
British Neuroscience Association / Gatsby Foundation Introductory Course on Glial Cells
British Neuroscience Association Festival of Neuroscience
Date: 11-14th April 2021
Brighton, UK
Aurox Conference on Microscopy 20201
Date: 7-8 April 2020.
Location: Virtual conference.
Link to Dr Fulton's talk
13th European Meeting on Glial Cells in Health and Disease (EuroGlia)
Date: 8-11th July 2017
Location: Edinburgh, UK
Daniel and Masa will be presenting new data from the STEM-ZAP project in the late-breaking abstract section.
British Neuroscience Association Festival of Neuroscience
Date: 10-13th April 2017
Location: Birmingham, UK
American Society for Neurochemistry 47th Annual Meeting
Denver, Colorado, 19th – 23rd March 2016
Poster presentation:
D. Fulton, U. Ahmed, Z. Ahmed, A. Stevens, G. Begum
Excitotoxicity: identifying novel targets for the protection of oligodendrocytes
12th European Meeting on Glial Cells in Health and Disease (EuroGlia)
Bilbao, Spain, 15th to 18th July 2015
Poster presentation:
G. Begum, U. Ahmed, A. Stevens, D. Fulton
Transcriptional regulation of AMPA-type glutamate receptors in the oligodendrocyte lineage
International Society for Neurochemistry Biennial Meeting
Cancun, Mexico, 20th – 24th April 2016
Poster presentation: D. Fulton
Neuronal activity and AMPA-type glutamate receptors regulate oligodendrocyte precursor cell process morphology and proliferation
The Oligodendrocyte & Myelin Research Group has developed an interactive workshop, ‘Why White Matter Maters’, which introduces school students to neuroscience, and in particular the important role that white matter (myelinated CNS structures) plays in health and disease. Through the workshop the students discover the divisions between grey and white matter in real brains, run an experiment that illustrates the impact of myelin injury on the speed of nerve impulses, and learn how the spiral structure of myelin is layered onto an axon through a game involving party whistles! We have run this workshop at numerous events including ‘Insight / Discovery: Medicine days, where GCSE students are introduced to biomedical research, and the University of Birmingham’s ‘Community Day’ which provides children of all ages with the opportunity to participate.

Dr Fulton was a member of the local organising committee for the BNA’s Festival of Neuroscience public events. His involvement included organising the following activities within the Arts Council funded ‘Fun and Brains’ events:
‘How does your brain work?, a neuroscience talk aimed at primary school children based on Stacey Bedwell’s recent book of the same title.
‘Ageing and Mindfulness’ – A talk from mindfulness author Adam Ford based on his book “Galileo and the Art of Ageing Mindfully” (organised in cooperation with Birmingham’s 1000 Elders research cohort).
Dr Fulton and Mo Sayed, also acted as scientific advisors / scriptwriters for ‘Build-a-brain’, a workshop developed and presented by Artists We are Frilly where children learnt basic principles of neural circuits through the act of crafting different types of neurons. Members of the research group including Masa Otsu, Simon Foale and Mo Sayed also helped to present this activity, helping children and their parents to construct neurons from recycled materials donated from local engineering firms.

